Palmer amaranth is a highly competitive weed of field corn, peanut, soybean and, especially, cotton. Biotypes resistant to glyphosate have been confirmed throughout the SE USA; in 2012, glyphosate-resistance in Palmer amaranth is suspected to occur in the Southern San Joaquin Valley here in California.
Herbicide resistance can develop de novo in plant populations via spontaneous genetic mutation, meiotic recombination and transposable element activity. Herbicide resistance can also be acquired via gene-flow, which is achieved through the movement of seeds and pollen. For species like Palmer amaranth that are dioecious (i.e. produce male and female flowers on separate plants), and/or produce seed that lack specialized dispersal structures, pollen-flow can be crucial for maintaining genetic diversity and disseminating beneficial alleles.
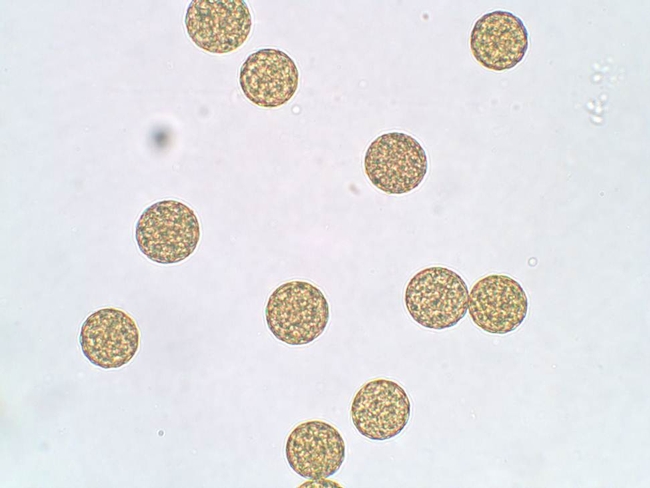
The pollen of Palmer amaranth is small (between 20-40 micrometers in diameter, with an average diameter of about 25 micrometers) and spheroidal and covered with shallow pores (Figure 1).
Figure 1. Palmer amaranth pollen as viewed using a light microscope. Mean pollen diameter was approximately 25 micrometers. For comparison, corn pollen is approximately 90 micrometers in diameter. A dime is approximately 17,910 micrometers in diameter.
The probability of a successful germination event occurring is dependent on how long pollen grains survive in the atmosphere after they have been shed; environmental factors such as temperature, relative humidity, UV radiation and atmospheric pollutants can significantly affect the lifespan of pollen. Therefore, a small, exploratory study was conducted at the University of Georgia in 2007 to describe how Palmer amaranth pollen viability changed over time in response to environmental conditions.
For five days in July and August of 2007, freshly harvested pollen grains (from naturally dehiscent anthers) were dusted onto microscope slides and exposed to local atmospheric conditions (Figures 2 and 3) for up to four hours. Samples were brought back into the lab at regular intervals and stained with tretrazolium bromide (MTT), a yellow liquid that is enzymatically (via dehydrogenase) converted into a reddish-purple crystal in living cells. We evaluated color change by capturing images of the pollen grains (no less than 300 for each sampling period each day) and used color analysis software to describe the pigmentation in the pollen grains with respect to the amounts of red, green and blue present. Freshly harvested and enzymatically active pollen grains should stain more darkly whereas older pollen grains should stain more lightly.
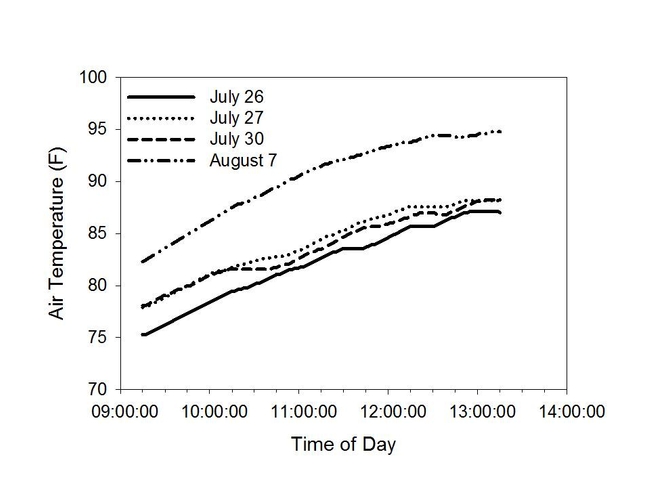
Figure 2. Air temperature (F) for the days when the study was conducted.
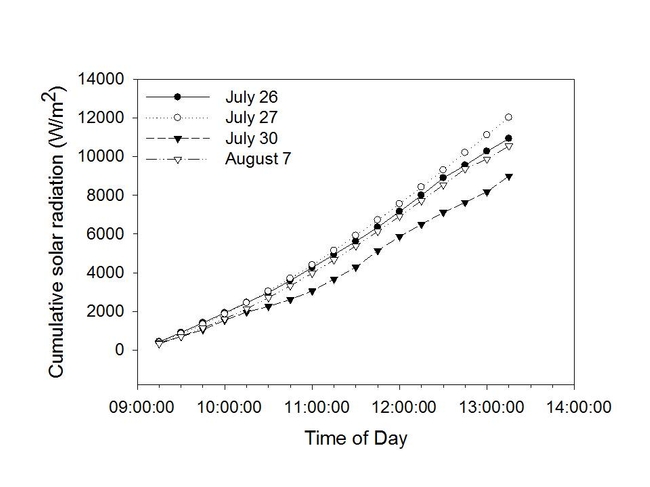
Figure 3. Solar radiation (W/m2) for the days when the study was conducted.
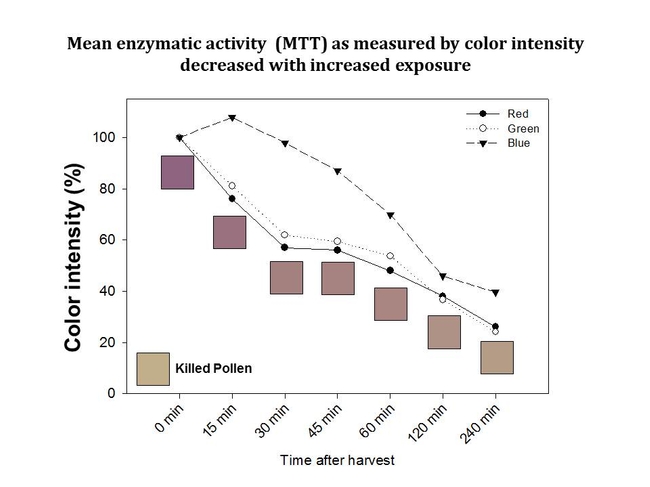
Results from our study showed that the degree of color intensity (and, therefore, dehydrogenase activity) decreased with increased time, post-harvest (Figure 4). Reductions in color intensity were associated with increasing daytime temperatures (Figure 2) and cumulative solar radiation (Figure 3).
Figure 4. Changes in dehydrogenase activity in Palmer amaranth pollen over time as determined by measuring color intensity.
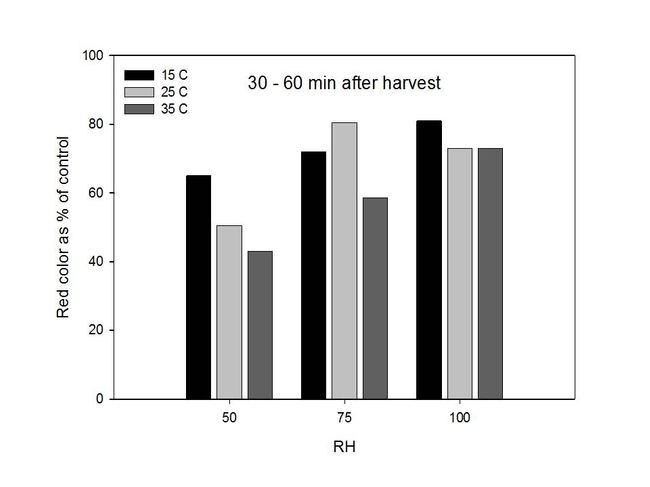
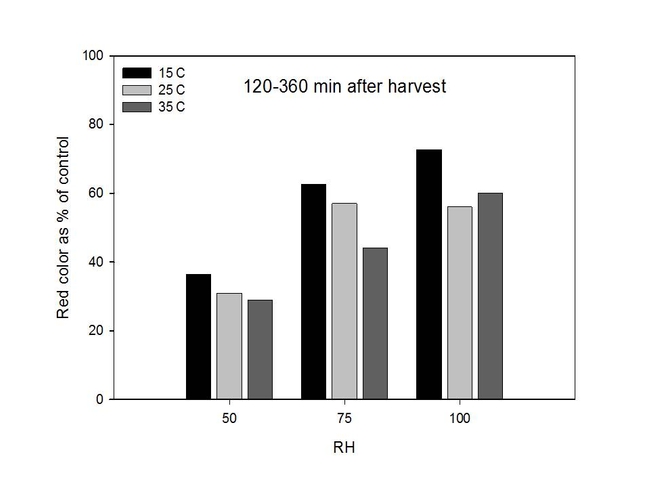
In a second series of studies, we stored freshly harvested Palmer amaranth pollen at specific temperatures (15, 25 and 35 C) and RH (50, 75, and 100%) for 30, 60, 120, 240 and 360 minutes; all studies were conducted in growth chambers. Pollen were stained and evaluated as described previously. In general, results from the growth chamber experiment showed that dehydrogenase activity ("stainability") in pollen grains tended to decrease when pollen was exposed to higher temperatures and lower relative humidities for longer periods of time (120-360 minutes versus 30-60 minutes). For example, pollen color intensity was usually lower for pollen kept at 35 C than 25 C and 15 C. Similarly, color intensity was reduced at 50% RH as compared to 75% RH and 100% RH (Figures 5 and 6).
Figure 5. Changes in dehydrogenase activity in Palmer amaranth pollen over time as determined by measuring color intensity.
Figure 6. Changes in dehydrogenase activity in Palmer amaranth pollen over time as determined by measuring color intensity.
Because we only measured the activity of one enzyme (and only one population of Palmer amaranth), it is impossible to say exactly how long the pollen grains remain viable in the atmosphere. A study conducted in the Midwest found that the pollen of common waterhemp (Amaranthus tuberculatus), another dioecious amaranth, could remain viable for up to 120 hours. One thing is certain, though: to prevent the buildup of excessive Palmer amaranth populations in agricultural and orchard systems, and limit the spread of undesirable traits, growers should not allow Palmer amaranths to achieve reproductive maturity!