Posts Tagged: ISHB
Beetle:Fungus Symbiosis?
Origin and evolution of fungus farming in wood-boring Coleoptera – a palaeontological perspective
David Peris, Xavier Delclòs, Bjarte Jordal https://doi.org/10.1111/brv.12763
A beetle bores a tree trunk to build a gallery in the wood in order to protect its lay. As it digs the tunnel, it spreads ambrosia fungal spores that will feed the larvae. When these bore another tree, the adult beetles will be the transmission vectors of the fungal spores in another habitat. This mutualism among insects and ambrosia fungi could be more than 100 years old --more than what was thought to date-- according to an article published in the journal Biological Reviews.
The study analyses for the first time the symbiotic associations and the coevolution between ambrosia fungi and beetles from a paleontological perspective using the Cretaceous fossil records of these biological groups. Among the authors of the study are the experts David Peris and Xavier Delclòs, from the Faculty of Earth Sciences and the Biodiversity Research Institute of the University of Barcelona (IRBio), and Bjarte Jordal, from the University of Bergen (Norway).
Beetles that grew fungi millions of years before human agriculture
Some termites, ants and beetles developed the ability to grow fungi in order to eat millions of years ago. This mutualism between insects and fungi --one of the top studied symbiosis in the natural field-- is an analogous evolutionary strategy in the farming activities of the human species since the Neolithic revolution.
Understanding the origins of the symbiosis between insects and fungi is a field of interest in several scientific disciplines. Nowadays, the mutualism between ambrosia symbiont beetles and fungi is the cause of forest and crop plagues that cause serious ecological and economic losses "it remains unclear which ecological factors facilitated the origin of fungus farming and how it transformed into a symbiotic relationship with obligate dependency", notes David Peris, first author of the study.
When did the lineage of farming insects begin?
Historically, phylogenetic studies suggest beetle fungiculture started more than 50 million years ago --before other insects-- and some studies dated it back to 86 million years ago. "The symbiotic relationship between fungus and beetles would have probably originated more than 100 million years ago, during the early Cretaceous, in groups of beetles that had gone unnoticed", reveals the expert David Peris.
As part of the study, the experts studied several specimens of worldwide distribution of the biological groups captured in amber from the Cretaceous. Therefore, the origin of ambrosia fungus is older than the main groups of beetles from the subfamilies Scolytinae and Platypodinae --Curculionidae family-- which now grow fungus in tree trunks, as stated by the authors.
"This suggests that these fungi used some other group of insects to spread millions of years ago", notes the researcher. Also, other beetle groups with a similar behaviour to ambrosia beetles --Bostrichidae and mostly Lymexylidae families-- present an older and abundant fossil record that would coincide with the emergence of ambrosia fungi, according to previous studies.
"The most interesting thing --he continues-- is that some studies note the ability to cultivate fungi in some of these current species".
Evolutionary convergence towards an obligate mutualism
The growing process of fungi starts when beetles colonize a new tree trunk or branch. During the Cretaceous, the abundance of fungi and wood-boring beetles facilitated a starting domestication of some groups of fungi. First, the fungal spores were accidentally transported from tree to tree by the wood-boring beetles "until this mutually beneficial association evolved towards a more intimate symbiosis in which fungi were inoculated into to a tree, the fungal mycelia grew and beetle larvae fed from the fungus", notes Bjarte Jordal.
This set of factors, together with the symbionts' high ability to adapt and change, eased the morphological and ecological adaptations of biological groups that converged in an obligated mutualism. That is, a symbiotic relationship between insects and fungi, beneficial for both, which still lasts.
"However, we need more studies on the knowledge of the ecology of the species from the Lymexylidae and Bostrichidae families to get more specific conclusions. Therefore, the discovery of new fossils in cretaceous amber of these groups will certainly help us to better understand the evolutionary history of this symbiotic relationship that still exists nowadays", concludes Professor Xavier Delclòs.
Read on: https://onlinelibrary.wiley.com/doi/full/10.1111/brv.12763
Photo: Invasive Shot Hole Borer tunnels in Box Elder trunk

shot hole borer feeding
Shot Hole Borer in the Tijuana River?
This is the most recent status report of theKuroshio Shot Hole Borer in the Tijuana River Valley of San Diego. For the full report go to: https://trnerr.org/kshb/
The Ecology and Management of the Kuroshio Shot Hole Borer
in the Tijuana River Valley
2019-20 (Year 5)
By
John Boland and Kellie Uyeda
This report presents the current status of the Kuroshio Shot Hole Borer (KSHB, Euwallacea kuroshio, Coleoptera: Scolytidae) in the Tijuana River Valley. It provides current rates of KSHB infestation; documents the current state of post-KSHB recovery in the most impacted forests in the valley; compares the current data with data collected over the past five years; and uses GIS technology for the first time to accurately map the spread of the KSHB in the valley.
This report is the fifth in a series of annual reports about the KSHB in the valley. It adds to and further develops four main storylines about the KSHB in the valley:
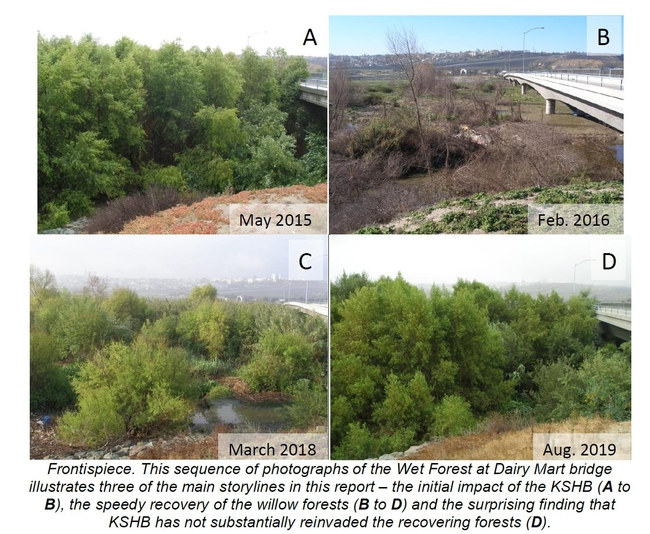
1. The KSHB in the valley went through a rapid boom-and-bust cycle. Annual surveys of infestation rates in the field and annual calculations of canopy damage from satellite images show that the KSHB population went through a rapid outbreak and a rapid decline over a five-year period. The infestation rates peaked in Fall 2016 and the canopy damage was greatest between 2016 and 2017. The early increase in population was characterized by the KSHB's presence in the Wet Forests and the swift damage to these forests (see frontispiece). The later decrease in population was characterized by the KSHB's presence in the Dry Forests and the slower damage to those forests. The KSHB population decline appears to be due to the KSHB depleting their preferred host trees and not reinvading the recovering host trees in the Wet Forests. This boom-and-bust cycle occurred naturally, with no management interventions to control the spread or severity of the outbreak.
2. The willow forests that were extensively damaged by the KSHB are responding with vigorous regrowth. Since the KSHB heavily damaged the Wet Forests in 2015-17, there has been extensive willow forest recovery in three ways: by the survival of a few, scattered mature infested trees (‘Big Trees'); by the resprouting of mature KSHB-damaged trees (‘resprouts'); and by the seeding of new trees (‘seedlings'). The frontispiece shows the striking recovery of one of the Wet Forests. Some of the forests have recovered so much in just four years that they are now similar to their pre-KSHB stature.
3. The KSHB has not substantially reinfested the recovering willow forests. Even though others predicted that all of the trees in the recovering Wet Forests would be quickly reinfested, only 3% of the Big Trees, 2% of the resprouting trees, 1% of the young trees, and 0% of the seedlings were infested with KSHB in 2019. This unexpected result begs the question: Why are the recovering willows not being attacked by the KSHB? In this report we suggest three possible reasons.
4. The invasive plant, Arundo donax, is now a major problem. Willow trees are arundo's only competitors in the valley, and when the KSHB attacked and heavily damaged the willows it allowed arundo to flourish more than ever before. Our main recommendation for the park managers in the valley is to control the arundo on their property. To assist them we provide a map of the current distribution of arundo using satellite images and object-based imagery analysis (OBIA) software.
The research reported here is unique among KSHB studies. It involves detailed and long-term field surveys of the KSHB invasion in one valley, documents an entire boom-and-bust outbreak of the KSHB and describes the damage to and recovery of the forests. This report also discusses the six most important ecological findings and suggests that incorporating these findings into the existing predictive numerical model would make the model more accurate. Finally the report presents several recommendations for needed future research and for immediate management actions.
Further Project Summary and Comments about Invasive Species: http://nivemnic.us/
kshb willows in Tijuana river
Shot Hole Borer in Avocado - Webinar
What Are the UC Ag Experts Talking About?
Invasive shot hole borers in avocado
May 20, 2020, Wednesday, 3-4 PM
Upcoming topics:
- Vertebrate pests by Roger Baldwin (June 2020)
- Ants in citrus by Mark Hoddle (July 2020)
- Use of plant growth regulators on citrus by Ashraf El-kereamy (August 2020)
Check out previous talks and listen to their recordings

shot hole borer
Alcohol Loving Ambrosia Beetles
Ambrosia beetles, which are a large group of several thousand species worldwide, belong to the bark beetles. All species are characterized by the ability to cultivate fungi. Invasive Shot Hole Borers that attack avocado and a range of native and landscape trees in California and the Laurel Wilt Disease that hammers avocado in Florida are ambrosia beetles. These beetles cultivate fungi in living trees and over time, the fungus is what kills the tree.
Beetles share the work of cultivating their fungal gardens: some clean the tunnel systems that are being eaten into the wood; others clear the dirt from the nest and clean their fellow workers -- always with the aim of optimizing the symbiosis of beetle and fungus.
It's been long known that alcohol is produced by weakened trees and that these trees are recognized and colonized by the beetles. Traps baited with alcohol are used to catch the insects when they fly. Alcohol is very attractive to the beetles in large part because the fungi they feed on performs best in an alcohol-rich environment. Alcohol is normally used as a preservative to impede other fungi, such as molds from growing, and this is the case for the fungi associated with these beetles. They prefer to grow in an environment where other fungi don't grow.
Here's an interesting article showing how this preference by disease-causing fungi allows them to thrive in a normally harsh environment. Maybe it can be exploited.
Christopher M. Ranger el al., "Symbiont selection via alcohol benefits fungus farming by ambrosia beetles," PNAS (2018). www.pnas.org/cgi/doi/10.1073/pnas.1716852115
Photo: Party Beetles
Credit: Gernot Kunz

ambrosia beetles
Tea Shot Hole Borer - PSHB, KSHB, ISHB
A “boring” problem that's generating
big interest in southern California
By Deena Husein, UCR Entomology, Ph.D. Candidate, Stouthamer Lab, UC Riverside
It has been a little over a decade since the first sighting of the polyphagous shot hole borer (PSHB) in Los Angeles County, yet a reliable control method has not been established. Part of the setback was caused by the misidentification of the species due to the beetle having a similar appearance to a low-risk pest — the tea shot hole borer. Aside from Los Angeles County, the PSHB is now established in Ventura, Orange, Riverside, San Bernardino and Santa Barbara Counties. We should be very alarmed at the rate the beetle has spread in southern California! The PSHB bores tunnels, also called galleries, in the xylem of the plant host and compromises the water transportation system. Tissue damage on the plant host is not only caused by the boring action alone, but also by the symbiotic, yet pathogenic, fungi the beetles carry to inoculate the gallery walls and utilize as a food resource. Consequently, the fungal buildup obstructs more water from reaching the branches, which can lead to dieback symptoms and eventually the mortality of the tree. The underlying issue lies in the gradual increase of plant species susceptible to being attacked. Currently, there are over 300 plants, 60 of which the beetle can reproduce on, including several native oaks, maples, sycamores, and willows along with ornamental plants and major agricultural crops (avocado) (Eskalen et al. 2013). A study by McPherson et al. 2017 showed that 23.2 million trees (32.8%) of southern California's region are susceptible to attacks by the PSHB beetle. Should half become infested, the approximate cost of removing and replacing those trees will be $15.9 billion with an annual accruement of $616.9 million over the next 10 years. The damage and loss the beetles have already costed the state will only continue to rise without the establishment of an effective management program.
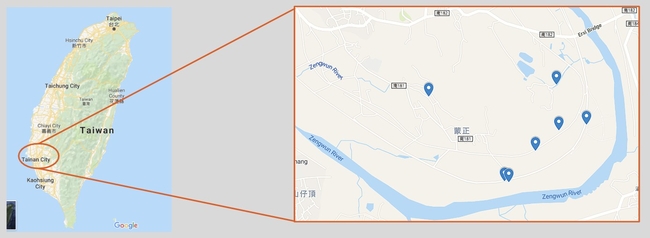
We understand that this pest is a major threat to the forestry and agricultural industry, but what efforts have been made to stop it? So far, conventional methods such as pesticides and mate disruption through pheromone traps have been rendered ineffective due to the beetle's cryptic lifestyle. The PSHB mates prior to leaving its natal gallery and is only outside for a brief period of time spent entirely on finding a suitable plant host. With that in mind, our laboratory has taken a different course of action that focuses entirely on biological control. Analyses of beetles sampled worldwide paved the path to a foreign exploration trip to Taiwan in the hopes of finding promising natural enemies of the PSBH. Since we are dealing with a wood boring beetle that remains hidden inside of its host, the best approach to encounter any natural enemy is by collecting beetle-infested plant material such as logs. The collection sites can be seen on the map below, all of which were in the district of Tainan City. Infested logs were shipped to the Insectary and Quarantine facility located at the University of California, Riverside (Figure 1A). From there, logs were individually placed in separate containers and monitored daily for anything that emerged (Figure 1B). After three trips and many logs later, we were able to identify three promising parasitoid wasps and two fungal-feeding nematodes associated with the PSHB.
The first parasitoid wasp we have encountered belongs in the family Bethylidae (Figure 2). A closely related species in the same genus has been documented to parasitize the coffee berry borer, another invasive pest with a similar lifestyle to the PSHB. While breaking apart some of the logs, we were able to find parasitized beetles with silk cocoons extending from their heads. Inside one of these cocoons was a bethylid pupa, which looks very promising (Figure 3). The second parasitoid is a braconid with an interesting method of attack. This novel species in the genus Sinuatophorus has prominent and large mandibles to 1) remove any excess material, such as frass and sawdust, at the entrance of the beetle gallery then 2) clasp the head of the beetle and eventually parasitize it. However, most taxonomic descriptions of wasps in this genus always cite Seitner and Notzel's publication from 1925, which mainly focuses on the development and lifecycle of another closely related wasp in a different genus. This poses as a challenge since our understanding of the lifecycle of this novel wasp is limited. Despite this setback, we were able to rear 4 generations by reintroducing every newly emerged wasp to multiple PSHB-infested castor bean logs.
The third parasitoid belongs to a very small, yet unique, group. Not only does this wasp attack beetles in their adult stage, but it also lays two eggs: one male and one female. Once fully developed, the female chews a hole from the inside of the abdomen and creates an exit path for herself and the male wasp. But the excitement doesn't stop there! While rearing beetles that emerged from the shipped logs, we were able to come across multiple nematodes. While most of them appeared harmless, some were categorized as fungal feeders. By introducing these fungal-feeding nematodes to beetle galleries, we could potentially use them as an indirect approach to mitigate the population of the beetles by attacking their food supply.
We have come pretty far in the past three years and are now able to narrow our efforts on these natural enemies. Though, as is the case with most biological control programs, there are many challenges and failures that will be faced. Our biggest hurdle to overcome is the cryptic lifestyle of the beetle. Once we introduce newly emerged wasps to beetle-infested logs, any control we have over the rearing process is taken away. Basically, this becomes a black box with no way of observing any parasitization occurring inside without breaking into the logs and jeopardize killing the insects by accident. We are currently trying to improve our rearing methods in the hopes of increasing our wasp colony size. Eventually with large numbers, we can move forward with non-target testing and come to a decision on whether or not we can utilize these natural enemies as a biological control agents to suppress the PSHB population.
Map: collection sites of PSHB beetle-infested avocado logs from Tainan City, Taiwan.
Figure 1A (left): Bag with beetle-infested avocado logs from Taiwan. Figure 1B (right): Temperature-controlled room with logs separated in plastic containers in the Insectary and Quarantine facility at UCR.
Figure 2:Image taken of Bethylid adult that emerged from beetle-infested avocado logs collected from Taiwan. Credit: Iris Chien
Figure 3 (top): Parasitized PSHB found with a silk cocoon extended from the head of the beetle. Bottom:A cocoon was dissected, adjacent from the intact cocoon, with a visible bethylid pupa.
Read and see more at:
http://ceventura.ucanr.edu/newsletters/Topics_in_Subtropics80946.pdf
Literature Cited:
Eskalen, A., Stouthamer, R., Lynch, S. C., Rugman-Jones, P. F., Twizeyimana, M.,
Gonzalez, A., and Thibault, T. (2013). Host range of Fusarium dieback and its
ambrosia beetle (Coleoptera: Scolytinae) vector in Southern California. Plant Dis,
97(7), 938–951.
McPherson, G., Xiao, Q., Van Doorn, N. S., De Goede, L., Bjorkman, J., Hollander, A., Boynton, R. M., Quinn, J. F., and Thorne, J. H. (2017) The structure, function and value of urban forests in California communities.Urban Forestry & Urban Gardening 28: 43-53.
Seitner, M. and Notzl, P. (1925). Pityophthorus Henscheli Seitner und sein Parasit
Cosrnophorus Henscheli Ruschka.