
Posts Tagged: Verticillium
Year One of a Test of Biological Fungicides in Strawberries.
This is simply a summary of one year of biological fungicide work in strawberries in 2012-2013 and should not be understood as a recommendation to use any of these products. This investigation is will continue into 2013-2014 and will serve to confirm and adjust the work here.
Introduction: A number of biological fungicides registered for use in strawberries have not been thoroughly tested through empirical studies to give guidance to growers on their efficacy and use.
Materials and Methods:
Table 1 below is an outline of materials tested in 2012-2013.
The field involved in the study was organically farmed and had a tested infestation of Verticillium at the average level of 25 microsclerotia per treatment replicate. Grower standard was managed as per grower practice, that is to say normal irrigation, fertility and pest management practices were applied. This grower standard did not include any sort of biological fungicide either by dip or injection through the drip tape during the season.
Plot was replicated three times and each replicate consisted of at one bed of 180 feet in length.
Application of the materials took place as indicated in the table below:
Table 1. List of treatments.
|
Test Material |
Application/ Use |
|
Dazitol |
6.25 gal/A applied 3-5 days prior to planting |
|
Biotam + Serenade Soil |
5 lbs/A pre-plant application 3-5 days prior to planting followed by Serenade Soil @ 4 qt/A (10 days after planting, and then Serenade Soil @ 2 qt /A applied monthly after planting. Second application of Biotam 5 lbs/A and Serenade Soil @ 4 qt/A in February. |
|
Serenade Soil |
Serenade Soil @ 4 qt/A (10 days after planting, and then Serenade Soil @ 2 qt /A applied monthly after planting. |
|
Serenade Soil |
Serenade Soil @ 4 qt/A (10 days after planting, and then Serenade Soil @ 4 qt /A applied every 60 days after planting. |
|
Actinovate Rate 1 |
(1) 3 oz per 100 gal root dip at planting. (2) followed by 6 oz/A pre-plant in drip tape (3) followed by 3 oz/A every 30 days in drip tape
|
|
Actinovate Rate 2 |
(1) 3 oz per 100 gal root dip at planting. (2) followed by 6 oz/A pre-plant in drip tape (3) followed by 6 oz/A every 30 days in drip tape
|
|
SoilGuard |
Apply at planting as root dip or planting furrow drench at 5 lb/ A and again through drip tape every 4-6 weeks through harvest. |
|
Double Nickel 55 |
Apply at planting at 1 qt /A (1/2 lb/A powder) as root dip or planting furrow drench and again through drip tape every 4-6 weeks through harvest. |
|
Terra Clean 5.0 |
(1) 128 fl oz/100 gal drench (2) 2 gal/A drip applied at planting (3) 1 gal/A drip applied 10 days post plant and 28 days post plant (4) 1 gal/A drip applied 60, 90 and 120 days post plant |
|
Terra Clean 5.0 + Serenade Soil |
(1) 128 fl oz Terra Clean /100 gal drench + Serenade Soil @ 6 qt/ A soil drench (2) 2 gal/A Terra Clean drip applied at planting followed by SS @ 3 qt /A (3) 1 gal/A Terra Clean + 3 qt /A Serenade Soil drip applied 10 days post plant and 28 days post plant (4) 1 gal/A Terra Clean + Serenade Soil @ 3 qt/ A drip applied 60, 90 and 120 days post plant |
|
Tainio |
(1) Spectrum @ 50 g / A + Pepzyme C @ 12.5 oz/A 2- 3 days preplant (2) Biogenesis @ 1 lb/A+ Pepzyme C @ 12.5 oz/A as plant dip (3) Pepzyme C monthly through drip tape (4) Micro 5000 @ 2.66 oz/A at 2 leaf stage foliar |
Plant dips were made by suspending the requested rate of fungicide in approximately twenty gallons of water and submerging and soaking about a half a box of strawberry transplants (about 500 plants) thoroughly and then distributing to planting crews for transplant. Note that in the case of the two Actinovate treatments, plants were held for one night to prior to transplant, ostensibly to establish the organism on the plant roots.
Drip applications were made with a portable pump injecting each fungicide. Each application normally was preceded by filling the drip tape with clear water, injecting the mix and then further pumping in clear water to make sure the fungicide had moved well out of the drip tape.
Application dates:
Preplant application – 11/3/2012
Dazitol
Terra Clean 5.0
Terraclean 5.0 + Serenade Soil
Tainio Spectrum + Pepzyme C
Biotam
Root dip + one overnight hold – 11/7/2012 (planted November 8)
Actinovate rate 1
Actinovate rate 2
Root dip and immediate planting – 11/8/2012
Double Nickel 55
Soilguard
Biogenesis + Pepzyme C
10 days post – plant – 11/20/2012
Serenade 4 qt
Serenade 2 qt
Monthly applications (12/11/2012, 1/16/2013, 2/26/2013, 3/27/2013, 4/30/2013, and 6/13/2013)
Terra Clean 5.0
Terraclean 5.0 + Serenade Soil
Tainio Spectrum + Pepzyme C
Serenade 4 qt
Serendade 2 qt
Actinovate rate 1
Actinovate rate 2
Double Nickel 55
Biotam applied 2/26/2013; Serenade 4 qt per acre applied other dates in treatment
Soilguard
Several dying plants from different areas of the test plots were sampled in July to confirm that Verticillium was the cause of plant death.
As a gauge of plant vigor from each treatment, strawberry plant diameters were measured February 13, and April 13. Measurements were in centimeters and from twenty plants in each plot.
With the beginning of fruit ripening, fruit harvest was done weekly in each treatment replicate. On each pick date, fruit from each plot was weighed and counted.
Results:
|
|
Plant D cm 2/13/2013 |
Plant D cm 4/13/2013 |
Yield to 5/14/2013 |
April Total Yield g/plot |
|
Actinovate r1 |
14.05a |
16.25a |
3894.00a |
2175.00ab |
|
Actinovate r2 |
13.70ab |
16.42a |
3818.33a |
2428.00a |
|
Biotam |
11.47b |
15.77a |
2176.67b |
1377.00b |
|
Dazitol |
11.70b |
17.70a |
2505.67ab |
1568.00ab |
|
Double Nickel 55 |
12.65ab |
15.92a |
3084.00ab |
1637.00ab |
|
Serenade 4 qt +2 qt |
11.57b |
16.65a |
2853.00ab |
1471.67b |
|
Serenade 4 qt +4 qt |
12.42ab |
16.02a |
2621.00ab |
1459.67b |
|
Soilguard |
11.85b |
16.78a |
2993.33ab |
1652.33ab |
|
Tainio |
12.93ab |
16.95a |
3454.00ab |
2075.33ab |
|
Terraclean |
11.50b |
16.78a |
2284.00b |
1406.67b |
|
Terraclean + Serenade |
12.47ab |
17.11a |
2656.33ab |
1559.67ab |
|
Untreated grower standard |
11.70b |
17.90a |
2145.00b |
1189.00b |
Means followed by same letter do not significantly differ (P=.05, Student-Newman-Keuls)
|
|
May Total Yield g/plot |
June Total Yield g/plot |
July Total Yield g/plot |
Aug Total Yield g/plot |
Total Yield g/plot |
|
Actinovate r1 |
3741.67a |
2791.00a |
1902.33a |
140.64a |
16820.67a |
|
Actinovate r2 |
3069.67a |
3237.67a |
2812.33a |
149.35a |
17201.34a |
|
Biotam |
2254.00a |
3251.33a |
3402.00a |
503.14a |
14468.33a |
|
Dazitol |
2514.67a |
3075.33a |
3049.33a |
269.16a |
14625.00a |
|
Double Nickel 55 |
3038.67a |
2837.67a |
2573.67a |
203.64a |
15052.67a |
|
Serenade 4 qt +2 qt |
3244.67a |
3287.00a |
3623.67a |
371.49a |
16887.67a |
|
Serenade 4 qt +4 qt |
2959.67a |
3617.67a |
3236.33a |
362.02a |
16157.00a |
|
Soilguard |
3212.33a |
3331.67a |
2887.00a |
359.45a |
16352.33a |
|
Tainio |
3409.00a |
3687.33a |
3190.33a |
170.27a |
18145.00a |
|
Terraclean |
2496.67a |
2997.00a |
2750.00a |
125.01a |
13681.33a |
|
Terraclean + Serenade |
2544.00a |
2810.00a |
2230.00a |
191.78a |
13454.33a |
|
Untreated grower standard |
2812.00a |
4019.33a |
3800.00a |
306.15a |
16150.33a |
Means followed by same letter do not significantly differ (P=.05, Student-Newman-Keuls)
Plant diameters measured on February 13, 2013, were significantly larger in the low rate of Actinovate than the other treatments with the exception of the high rate of Actinovate, Tainio and Double Nickel 55.
Both rates of Actinovate realized higher fruit yield than all other treatments except for the Dazitol, in cumulative fruit yield, which included six weekly harvests, up to May 14, 2013. No further differences were realized between May and August.
As noted above, this field has a very high infestation of Verticillium and all plots began to experience pronounced plant dieback in June, with some 60-80% remaining alive or declining and by end of July, nearly all plants in all replicate plots had died. There was a trend for plants which had produced larger amounts of fruit in April and May to experience lesser fruit production lesser vigor and earlier dieback as the season progressed.
Conclusion
The results of this trial are encouraging. In the early part of the season up through the middle of May, several treatments had significantly higher amounts of fruits harvested than the grower standard. Nonetheless, none of the treatments provided sufficient protection to the plants to prevent an almost complete die out of the plants by August, effectively ending the season.
Verticillium Wilt in strawberries: California 2013 Update
Verticillium wilt continues to be one of the most potentially damaging diseases caused by soilborne pathogens in strawberries grown in California. Historically Verticillium was the primary target against which soil pathogen mitigation, i.e. pre-plant soil fumigation, avoidance and crop rotation, and breeding for plant resistance, in strawberries was been directed.
Verticillium wilt symptoms: Early symptoms consist of stunting, delayed development, and the yellowing of lower leaves. As disease progresses the older leaves wilt, dry up, and become brown; typically the younger, central leaves of the plant remain green until the plant dies and all foliage turns brown (Figure 1). In contrast to Verticillium wilt of other crops such as lettuce, vascular discoloration in strawberry crowns may be subtle or absent (Figure 2). Strawberry disease symptoms can be accentuated if the infected plant is subject to stress such as from environmental extremes or mite infestations.
The Pathogen: Verticillium wilt in strawberry is caused by Verticillium dahliae. The host range of this pathogen is quite broad, though in recent years researchers have found sub-groups within V. dahliae that have preferred hosts and therefore narrower host ranges. For strawberry growers, they should be aware that the Verticillium isolates that infect lettuce and artichoke also infect strawberry. Likewise, the strawberry isolates of Verticillium can infect lettuce and artichoke. Verticillium gets its name from the whorls of spore-bearing branches (phialides) that are visible when the fungus is viewed under a light microscope (Figure 3).
Verticillium forms a survival structure, the microsclerotium, which allows the pathogen to survive unfavorable conditions and persist between host crops (Figure 4). Microsclerotia are dense masses of thick, dark (melanized) cells that form inside host tissues and are released into the soil when crop residues break down and decay. Researchers have developed methods for measuring the population of viable microsclerotia in soils; such measurements (microsclerotia per gram of soil [ms/gram]) can give growers and others an estimate of potential threat to strawberry plantings. Strawberry has a very low threshold tolerance for Verticillium. A soil test result of 3 ms/gram likely indicates that some disease may develop on the subsequent strawberry crop. With a soil test result of 10 or more ms/gram, strawberry should probably not be planted unless soil fumigation is planned. The picture below is of a field with an average of 30 ms/gram at the end of July (Figure 5).
Disease Development: Verticillium microsclerotia germinate in the soil when activated by exudates from the host plant roots. Once penetrating the plant root, the fungus grows up into the xylem (the water conducting elements of the plant), degrading the cell walls and most likely releasing toxins. This type of colonization is called a systemic infection. Systemic infections interfere with the plant’s ability to conduct water. For this reason, infected strawberry plants will wilt during times of high water demand, such as during hot and dry weather, if the plant is improperly (too dry) irrigated, or if bearing a heavy fruit load. Diseased plants that show some dieback symptoms may recover if the stress conditions subside; however, such plants are not likely to be as productive as unaffected, healthy plants.
The pattern and distribution of Verticillium wilt disease in the field can be extremely variable and does not necessarily correspond with low spots, heavy soil, or improperly irrigated areas. In contrast, such field conditions may correspond with patterns of Phytophthora crown rot. Instead, many times Verticillium wilt affected plants can be found distributed all over the field, perhaps as individual plants or as patches of affected plants.
Managing Verticillium in Strawberry:
Plant Breeding for Resistance: Strawberry plants genetically resistant to V. dahliae are not yet available commercially. However, resistance should play a big role in the future mitigation of Verticillium wilt in strawberry, though development of completely resistant cultivars has not been easily attained. In a research report (California Agriculture, January/March 2010), it is pointed out that intensive selection for Verticillium resistance resulted in a few genotypes that demonstrated a great amount of resistance when inoculated with the pathogen; however, these selections suffered some yield loss under intense disease pressure. Furthermore, these highly resistant genotypes all expressed “substantial deficiencies for horticultural or productivity traits,” meaning they weren’t producing the quantity or quality of fruit that we have come to expect in California.
Even so, the University of California strawberry breeding program has made significant advances in improving genetic resistance to Verticillium. Starting in the late 1980s, less than 40% of the genotypes used in the UC breeding program had moderate tolerance to V. dahliae; twenty years later, between 80 and 100% of the genotypes used had such tolerance.
Soil treatments: Soil fumigation with a mix of methyl bromide and chloropicrin is usually recommended for conventional growers, but this plan of action is becoming quite expensive if not impossible under new regulations and limitations. Chloropicrin used alone is successful in disinfesting soils of Verticillium, as are mixes of 1,3 – Dichloropropene and chloropicrin (Telone C-35) but generally none of these have been shown to be as effective as methyl bromide and chloropicrin used together in clearing a soil of Verticillium pathogen.
An alternative soil treatment being tested and demonstrated in several commercial fields is anaerobic soil disinfestation (ASD). ASD works by inducing an anaerobic (oxygen-less) condition in soils that are amended with a carbon source. The resulting proliferation of oxygen-consuming microbes shifts the soil ecology and microbial diversity, resulting in suppression of pathogenic organisms. Researchers are continuing to develop and fine-tune this method.
Biofumigation is another soil treatment that can reduce Verticillium numbers in the soil. Broccoli crop residues release chemicals that both directly reduce Verticillium propagules as well as affecting the soil microbial diversity, which can suppress the pathogen. While mustards and other cruciferous plants show similar effects, broccoli appears to be one of the best choices for this soil biofumigation treatment. A crop rotation that includes broccoli will have the same suppressive effect, since the harvested broccoli florets are not needed for biofumigation to take place.
Sanitation: Being a soilborne pathogen, V. dahliae is readily spread between fields in mud and dirt adhering to equipment and vehicles. Growers should therefore beware of moving contaminated equipment from infested fields into “clean” fields. Because diseased strawberry plants are infested with microsclerotia, strawberry crop residues should not be moved into other fields.
Crop rotation: Crop rotation is a key IPM practice that will help lessen the threat from Verticillium wilt. Consecutive, back-to-back plantings of strawberry is a risky practice if the field has a history of Verticillium wilt and if effective fumigants are not used. Fields which have been recently planted to lettuce, artichoke, and Solanaceous family crops (potatoes, eggplants, and tomatoes) should likewise be avoided if Verticillium wilt has occurred and if soil fumigation is not implemented. Weeds that are hosts of V. dahliae may not play a critical role in disease development but should be controlled in any case.
Other alternatives: Another experimental alternative to chemical control is the use of organic substrates, such as coconut peels (coir) or peat moss, which are used as the rooting medium in place of the soil but are kept completely separate from the soil by cloth barriers. The substrates are poured into cloth-lined furrows that are constructed into the beds. The intent of this approach is to create a pathogen-free zone above the field soil. This method is still being tested.
Finally, there are a series of commercially available biological fungicides which purport to competitively exclude pathogenic fungi such as Verticillium from the surface of the root, or which produce toxins inhibitory to pathogen growth. These materials likewise still require research and demonstration of efficacy under field conditions.
The above has been a discussion the biology and management of Verticillium wilt disease in strawberry. There are pesticides mentioned for the management of Verticillium in this article. Before using any of these products, check with your local Agricultural Commissioner’s office and consult product labels for current status of product registration, restrictions, and use information.
(The authors thank K. V. Subbarao for assistance with this report.)
References:
California Agriculture, Jan/Mar 2010. http://californiaagriculture.ucanr.org/landingpage.cfm?article=ca.v064n01p37&fulltext=yes
UC IPM Online, strawberry Verticillium wilt. http://www.ipm.ucdavis.edu/PMG/r734100811.html
UC IPM, Guia para el manejo de las plagas, Fresas. http://www.ipm.ucdavis.edu/PDF/PMG/pmgstrawberry_espanol.pdf

Figure 1: Brown foliage and plant collapse caused by Verticillium wilt. Photo Steven Koike, UCCE.

Figure 2. Crown of Verticillium-infected strawberry showing lack of internal crown discoloration.. Photo Steven Koike, UCCE.
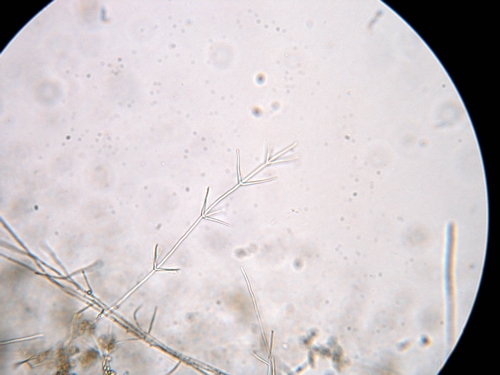
Figure 3. Microscopic view of the verticillate spore bearing structure of V. dahliae. Photo Steven Koike, UCCE.
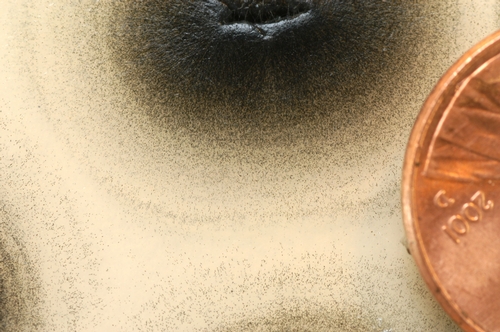
Figure 4. Culture petri dish showing profuse numbers of black microsclerotia. Photo Steven Koike, UCCE.

Figure 5. Significant dieback in a field highly infested (30 ms/gram) by V. dahliae. Photo Mark Bolda, UCCE.
Verticillium in Blackberry
A recent case of wilting blackberries around Watsonville has been confirmed from multiple samples submitted to several plant pathologists (the UCCE plant pathology diagnostic lab in Salinas obviously being one of them) to be caused by Verticillium dahliae. The pattern is one of wilted plants scattered over the breadth of the field, with very few groups of more than three dead or dying plants. Verticillium on blackberry is actually pretty rare to find; in my career of more than 10 years I have only found 4 other blackberry samples to be positive for this disease.
As one can see from the pictures below, the affected plants stand out from the rest of the field by having few to no leaves (Photo 1). Additionally, there are some leaves on affected plants which are a stark yellow color (Photo 2) and according to the literature this yellow leaf color can be diagnostic for Verticillium wilt. Note also the darkened color of the epidermis (Photo 3) and the obvious discoloration of the vascular tissue (Photo 4) found when cutting deeper into the cane.
Generally, it is not at all uncommon in this field to will find Verticillium infected branches and others which are apparently healthy on the same plant. This is consistent with an infestation of Verticillium which has not invaded all the roots and consequently has left some vascular tissue healthy and functioning.
The solution to this problem is to continue cropping if the disease does not manifest itself in many other plants with an eye to maintaining good watering practices so as to make up for the Verticillium compromised vascular system. However, when planting a new blackberry crop, it would is strongly suggested to fumigate or use a variety which is less susceptible to Verticillium disease.
Researchers have found that V. dahliae exists as a series of different strains that have different host preferences. Characteristics of the blackberry V. dahliae pathogen have not been studied. Until further research information is available, growers should therefore assume that V. dahliae from blackberry, raspberry, and strawberry all can cross infect these three crops. This assumption would be important to remember when considering crop rotations.

Affected blackberry.

Yellow leaves found on affected plants. No significant nutritional deficiencies in yellow leaves, other than a very high concentration of sodium.

Discoloration on epidermis of cane on blackberry.

Cutaway of affected cane.
Pest threats 'overblown'

The board agreed to revisit the 2008 regulation that prevents cotton growers from planting in the same field three years in a row if the level of verticillium wilt is detected in 3 percent or more of the crop.
Doug Munier, farm advisor for University of California Cooperative Extension, said verticillium dahliae exists in native valley soils and is spread by movement.
"Verticillium was a minor problem with cotton was first planted in the mid-1990s and it is still a minor problem after 17 years of production," he said.
New bee threat more media hype than reality
Wayne Anderson, The Washington Times
Government officials, leading bee experts and average beekeepers around the country say the strange discovery of a deadly bee parasite fly that makes honeybees act "zombie-like" is not seen nationwide — but only in a recently published San Francisco State University study. And some even question the veracity of the discovery itself.
The parasite fly is a long-time resident of California and a known nemesis to other bugs besides honeybees, the article said.
“They’ve been known to infest or parasitize bumblebees for some time all over the country,” said Eric Mussen, California State bee specialist and professor at the University of California, Davis. “It’s not new. We’re aware of it. I don’t believe it’s a terribly important thing to honeybees as a whole.”
Verticillium wilt showing up in Watsonville-Salinas strawberry fields
In the past two weeks, growers have been reporting strawberry field situations in which plants are not growing well, are falling behind in size and production, and are showing symptoms of collapse. Initially the older leaves lose their normal bright green color and begin to turn a dull gray green. These leaves later wilt, collapse, and become brown and dry. Early in disease development, the wilting leaves often occur only on one side of the plant. Without exception, the dead and dying foliage is restricted to the outer, older parts of crowns and the inner younger leaves remain symptomless. Examination of the plants showed that roots were normal and not diseased. The internal crown tissue is likewise healthy in appearance and not discolored. In affected fields, symptomatic plants are randomly scattered throughout large sections of the planting.
Plants submitted to the UC Cooperative Extension diagnostic lab (supported in part by the California Strawberry Commission) were tested for a wide range of pathogens. All such samples tested positive for the Verticillium wilt pathogen (Verticillium dahliae) and were negative for Macrophomina, Fusarium, or other pathogens.
The confirmation of Verticillium wilt is, of course, a major concern for growers. Presently the only effective management options are to rotate and plant strawberry in locations that do not have infested soils and/or to fumigate with effective materials. Because V. dahliae can survive in the soil for many years, even in the absence of a plant host, the disease is a long-term concern for growers. A major concern is that as our industry moves away from methyl bromide-chloropicrin pre-plant fumigation, these Verticillium wilt situations may become more common.
The Verticillium pathogen survives in the soil by producing microscopic, resilient resting structures called microsclerotia. Because microsclerotia are in the soil, growers should be reminded that the movement of significant amounts of infested soil (via mud clinging to equipment and vehicles or by in-field disking) will move the pathogen to uninfested locations. Researchers also find that microsclerotia can be found in high numbers in old strawberry crop residues. Note that the strain of V. dahliae that infects strawberry can infect other plants such as lettuce.

Photo Courtesy Steven Koike, UCCE. Overview of infested field.

Photo Courtesy Steven Koike, UCCE. Close up of infected plants.

Photo Courtesy Steven Koike, UCCE. Note wilting pattern of Verticilluim infected plant.
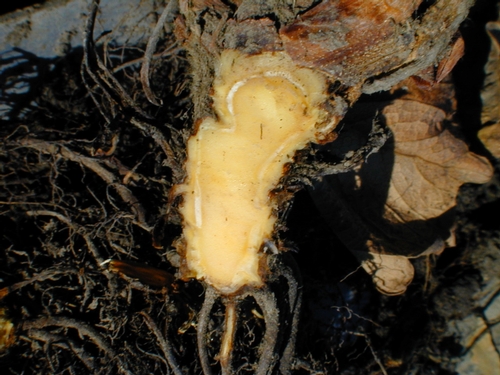
Photo Courtesy Thomas Flewell. Note how healthy the internal crown tissue appears, yet the plant was very much collapsed.
